

A multidisciplinary process development team undertakes phase-appropriate process R&D for customer molecules in the early phase of drug development stage. We address the key elements of route scouting, process optimization, process safety evaluation, HAZOP studies, backward integration and scalability, to deliver processes that are robust, safe, reliable and scalable.
Our team also conducts salt screening and polymorph evaluation in the very early stages of process development. Principles of phase-appropriate development and quality by design (QbD) are optimally used to support customer projects. We leverage our technical strength, analytical expertise and regulatory knowledge, to design and execute development strategies.
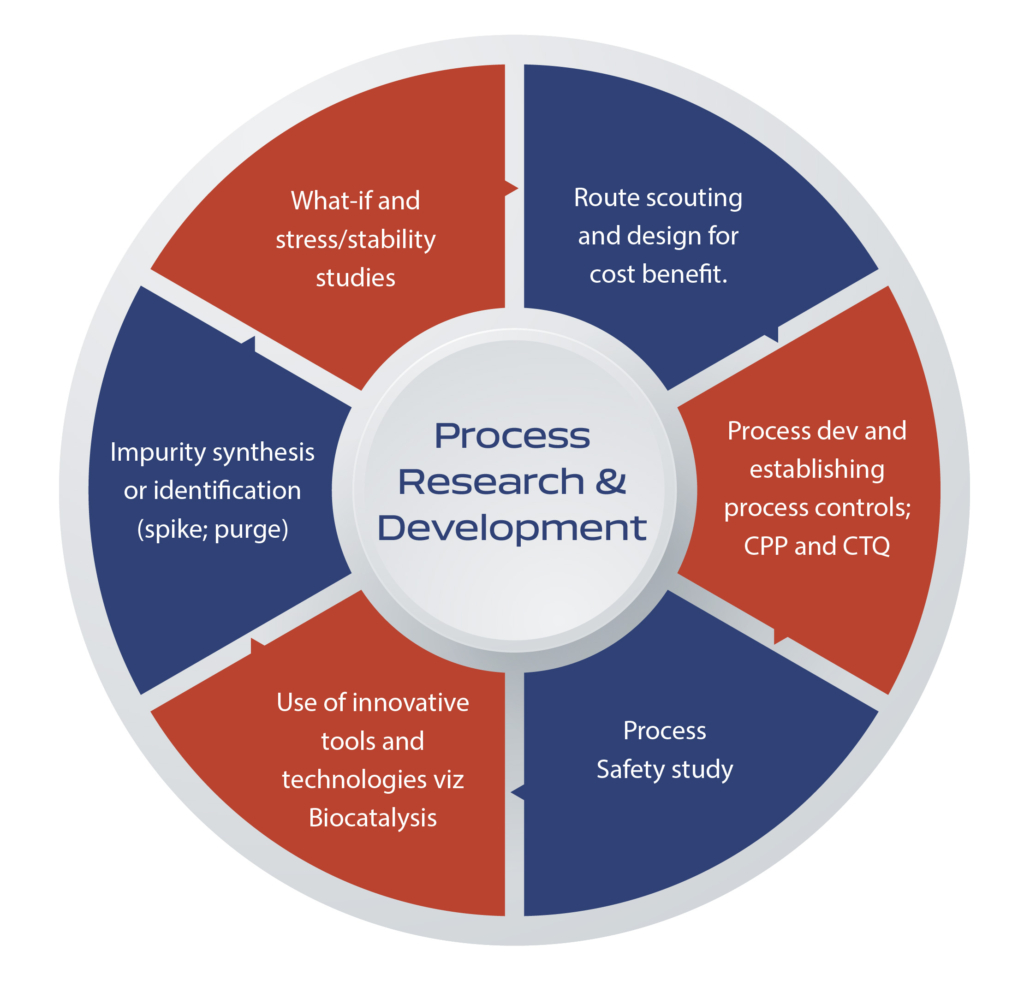
Process Research & Development Infrastructure
Route Scouting:
In the route scouting process, the goal is to generate multiple options and deliver a route that is suitable for IND and beyond, ensuring alignment with key agreed-upon criteria such as speed, risk, and economics. The proposed options should be flexible, allowing for adjustments based on varying resource levels. Integral to this process is the incorporation of QA standards within the evaluation to ensure the highest quality outcomes.
We use the SELECT principles of phase-appropriate development and quality by design (QbD) are optimally used to support customer projects. We leverage our technical strength, analytical expertise, and regulatory knowledge, to design and execute development strategies.
| 1 | Safety | The factors considered are stability of reaction, toxicity of the raw material used, any toxic gas/waste generated during the reaction or any unstable raw material used in the process. |
| 2 | Environment | Process research scientists strive hard to design a green route scouting method to reduce the waste generated, avoid toxic waste, and if the waste can be recycled. |
| 3 | Legal | Some raw materials such as narcotics or hazardous substances can be restricted, and IP can protect some steps required in the process. |
| 4 | Economic | Economic viability determines the steps and raw materials used in the process, minimizing the number of steps, developing telescopic processes, the availability of the raw materials in the domestic market, recovery of solvent, and cost associated with the disposal of the waste generated. |
| 5 | Control | Control related factors affect the deliverability of the project on time, in full, and with the highest purity. Process research scientists study and understand the parameters, flexibility of the process, and how to reduce the impurities. |

